Pfizer-BioNTech COVID-19 The vaccine has done little to protect inoculated children ages 5 to 11 from infection, according to data released by New York government officials on Monday.
Researchers from the New York State Department of Health assessed the degree of infection of minors who had Pfizer blow made available to them. The children were divided into two age groups, one for children aged 5 to 11 and the other for children aged 12 to 17.
They found that the two-dose Pfizer injection was only 12 percent effective in preventing infection in the younger age group just one month after receiving it.
The findings have far-reaching implications for the use of vaccines and whether parents will want to prick their children.
Children are not at high risk of the virus, and hospitalizations and deaths are particularly rare.
The main argument in favor of vaccinating them is to prevent the spread of the virus, although these findings suggest that the vaccine does little to prevent this anyway.
In cities like New York, five-year-olds were forced to show proof of a vaccine to enter indoor dining, fitness and entertainment venues. The children also had to be vaccinated in order to take part in some school activities.
This has probably influenced some parents’ decisions about the vaccine – as vaccination has been relatively low in younger age groups – and official government figures are now showing that the vaccine has done little to protect children. from infection.
Some parents also had significant fears about the potential side effects of the vaccine and risked giving their child the injection only to find that it was not potentially as effective as advertised.

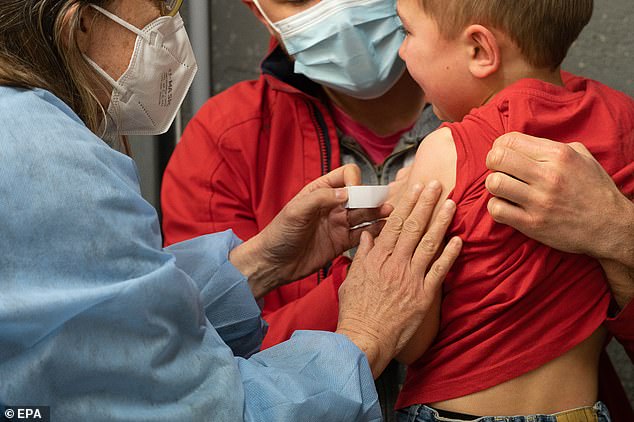
New York State officials said Monday that children aged 5 to 11 receiving the COVID-19 vaccine had a only 12 percent lower risk of infection. Pictured: A young child in France receives a vaccine against COVID-19
Researchers in New York collected data on 852,384 children aged 12 to 17 and 365,502 children aged 5 to 11 for the study.
The study that is prepress and pending peer review, collected data from the Omicron period of the pandemic, from December 2021 and January 2022.
Study participants were followed and compared with general data from unvaccinated populations.
One major difference between the study groups was the dosage of the vaccine. While children aged 12 and over receive a 30 microgram vaccine, it is only ten micrograms for the five to 11 age group.
The researchers found that 28 to 34 days after receiving the second dose – about a month – the effectiveness of the vaccine in the younger age group decreased from 67 percent to 11 percent compared to the variant.
This compares with a much smaller decline from 66% to 51% in the older age group.
There was also a significant difference found between 11 and 12-year-olds – the section where the vaccine dose was changed – suggesting that lower doses were less effective.
Pfizer has faced a similar problem with its dosage in children aged six months to four years.
For this age group, the stroke was reduced to three micrograms, although initial experiments showed that three- and four-year-olds had a small immune response to the smaller stroke.


Since then, the company has introduced a third blow to vaccine testing, and the FDA’s approval for the vaccine has been suspended as these data have been collected.
However, Pfizer is in trouble. The company wants to launch injections for younger age groups and has even been forced by regulators to apply before the third dose is available.
Adding millions of young children to extended vaccine eligibility would also make the New York-based company a good sum of money.
With the only photo available to people under the age of 18 in America, it has effectively squeezed an extremely lucrative market.
However, the mRNA technology used in the vaccine is now a problem.
Pfizer reduced doses in younger age groups to help understand the risk of vaccine recipients developing myocarditis.
The Centers for Disease Control and Prevention (CDC) has warned that recipients of vaccines distributed by Pfizer and Moderna – who also have Covid jab mRNA – are at risk of developing rare heart disease. This risk is especially prevalent in young men.


Although this did not cause as many Pfizer injections as Moderna – which has been restricted in some Scandinavian countries due to fears of heart inflammation – the company had to adjust its dosage levels to avoid an increased risk of young people.
This may have left children on the edge of any age group, those aged 11 and 4, with a vaccine that provides them with little in terms of vaccine protection.
“These results underscore the potential need to study alternative vaccine dosing for children and the continuing importance of multilayer protections, including wearing masks, to prevent infection and transmission,” the New York researchers wrote in the study.
Some questions about whether the vaccine is needed at all for younger children due to the extremely low risk they face from the virus.
According to the CDC, children have accounted for less than 0.1 percent of COVID-19 deaths in the United States since the pandemic began in March 2020.
A study from October last year also found that about half of Covid’s pediatric cases were asymptomatic, and that was before the milder version of Omicron became dominant in the United States.
When infected, children also release fewer virus particles into the air, making them less likely to spread the virus and study published by a German research team last week found.